Table of Contents
P450 and pesticide resistance
For recent and general reviews of the role of P450s in pesticide resistance, see Nauen et al., 2022 and Hayward et al. 2026.
A reminder of “resistance” definitions:
The classical definitions are from the WHO (1957): Resistance is the development of an ability in a strain of insects to tolerate a dose of toxicants which would prove lethal to the majority of individuals in a normal population of the same species; and from the FAO: resistance is the decreased response of a population of animal or plant species to a pesticide or control agent as a result of their application.
A Forum by Tabashnik et al. 2014 discusses critically the many ways “resistance” is defined, and proposes a general definition of resistance as a “genetically based decrease in susceptibility to a pesticide”. (italics are used here to point out that not all genetically based variation in susceptibility would qualify as resistance).
The fact that resistance is heritable and that it results at the population level from selection by a toxic agent is implicit in many definitions, yet of the essence of resistance. While many authors avoid or dislike the term tolerance, the definition of Finney (1971) is very useful: the highest concentration of a particular pesticide that an individual can withstand without being killed. It follows that natural or experimental variation in the toxicity of a pesticide is a measure of tolerance, not necessarily of resistance.
Phenotype, genotype and causal relationships
There are at least two ways of looking at resistance:
One way is to look at the phenotype: a biochemical or physiological change that causes resistance.
A second way is to look at the molecular genetic modification that leads to this phenotype.
The first way is the classical way whereby pesticide resistance is achieved in a selected strain or population (i) by an alteration of the target site (toxicodynamic change), (ii) by an alteration of the effective dose of insecticide that reaches the target (toxicokinetic change) or (iii) by a combination of the two. The resistance phenotypes have long been analyzed according to these useful biochemical and physiological criteria.
The second way documents at the molecular genetic level the classes of mutations that can account for these phenotypes (Taylor and Feyereisen, 1996; Feyereisen et al., 2015). These two dimensions of resistance are illustrated in this table, with checkmarks for cases documented by 2015. For P450-mediated resistance, focus is on toxicokinetics (middle column).
Different classes of mutations affecting a P450 gene and thus leading to resistance can be distinguished. They fall broadly in three categories as shown in the first column of the table above. The first are mutations affecting the coding sequence of the gene and thereby structurally alter the gene product. The second are mutations causing an increase in gene dosage or expression. The third are mutations causing a decrease in gene dosage or expression. The latter two classes can be further distinguished in mutations affecting the whole gene (such as duplication and amplification, or disruption and loss) or just the cis regulation or the trans regulatory elements of the gene.
Examples of all these precise molecular mutations responsible for P450-mediated resistance are known. The cis-mutation (Accord element insertion) in the 5‘UTR of the Cyp6g1 gene in Drosophila causing up-regulation of expression (Daborn et al., 2002) is one of the most detailed account.
Traditionally, the first line of evidence for a role of a P450 enzyme in resistance has been the use of an insecticide synergist (e.g. piperonyl butoxide, PBO)(Feyereisen, 2015). A suppression or decrease in the level of resistance by treatment with the synergist being diagnostic. In cases too many to list here, this initial and indirect evidence is probably correct, however there are cases where piperonyl butoxide synergism has not been explained by increased detoxification (Kennaugh et al., 1993). Piperonyl butoxide may also be a poor inhibitor of the P450(s) responsible for resistance, or may inhibit some esterase activity so that the use of a second synergist, structurally unrelated to PBO may be warranted (Brown et al., 1996, Zhang et al., 1997). In addition, the synergist as P450 inhibitor can decrease the activation of a proinsecticide, so that lack of resistance suppression can be misleading. An independent and additional line of evidence is the measurement of total P450 levels or metabolism of selected model substrates. An increase in either or both being viewed as diagnostic. Such evidence is indirect, and the absence of change is not informative.
An increase in the metabolism of the insecticide itself in the resistant strain is more conclusive and has been demonstrated in many studies. For instance, permethrin metabolism to 4'-hydroxypermethrin was higher in a microsomes from Culex quinquefasciatus larvae that are highly resistant to permethrin (Kasai et al., 1998b) than in their susceptible counterparts. Total P450 and cytochrome b5 levels were 2.5 times higher in the resistant strain. Both permethrin toxicity and metabolism were inhibited by two unrelated synergists, TCPPE and piperonyl butoxide. A similarly convincing approach was taken to show P450 involvement in the resistance of house fly larvae of the YPPF strain to pyriproxifen. Gut and fat body microsomes were shown to metabolize the IGR to 4'-OH-pyriproxyfen and 5“-OH-pyriproxyfen at higher rates than microsomes of the susceptible strains and this metabolism was synergist-suppressible (Zhang et al., 1998). The major, dominant resistance factor was linked to chromosome 2 in that strain (Zhang et al., 1997).
Increased levels of transcripts for one or more P450 genes in insecticide resistant strains has now been reported in many cases. These transcriptomic studies suggest that overexpression of one or more P450 genes is a very common phenomenon of metabolic resistance but they do not by themselves establish a causal relationship with resistance. Instead, they merely provide lists of candidate genes.
Genetic linkage between increased mRNA or protein levels for a particular P450 and resistance has been obtained to the chromosome level or closer to marker genes (Feyereisen, 2005; Bogwitz et al., 2005; Wee et al., 2008; Hardstone et al., 2010). Linkage is just the first step in establishing a causal link between a P450 gene and resistance. At a much finer scale, bulk segregant analysis (BSA) and quantitative trait loci (QTL) mapping of resistance, when experimentally feasible, provide rapid, non-biased approaches to candidate genes for resistance (e.g. Irving et al., 2012; Fotoukkiaii et al., 2021).
Functional expression of the P450 enzymes in heterologous systems (in vitro activity of the enzyme) is required to establish the contribution to resistance of a CYP gene. However, while necessary, this criterion is not sufficient. Indeed, several P450s can contribute to the metabolism of a chemical in susceptible strains, while perhaps just one of them (through expression or sequence change or both) is in fact responsible for resistance.
Transgenic expression in Drosophila or other insects followed by in vivo toxicity assays can provide convincing evidence, but the interpretation of such experiments is not always straightforward. For instance, while Bogwitz et al. (2005) demonstrated that Cyp12a4 overexpression in the midgut and Malpighian tubules confers lufenuron resistance in Drosophila, they also showed that high level ectopic expression of the gene was embryonic lethal (for an unknown reason).
CRISPR/Cas gene editing, such as gene knockouts or even knockout of gene clusters is an increasingly accessible and very effective technique to study the contribution of one or more P450 genes to xenobiotic metabolism.
RNAi suppression of CYP gene expression is very informative, but this technique is not equally effective in all insect species. Furthermore, the effects observed are often marginal.
Ultimately, a combination of genetic and biochemical evidence is needed to make a compelling case for the involvement of one or more P450s to resistance.
Types of resistance mutations
Several types of mutational events can lead to resistance, and these are described schematically below. It is common to see resistance caused by more than one type of mutation.
Mutations affecting the coding sequence of P450 genes
Structural changes in P450 proteins:
Mutation(s) (yellow triangle) in the open reading frame of the P450 gene can result in the production of a P450 with altered catalytic activity. The enzyme produced can have a higher specific activity towards a pesticide, and/or change the types of metabolites produced. Examples include CYP9A186-F116V in Spodoptera exigua.
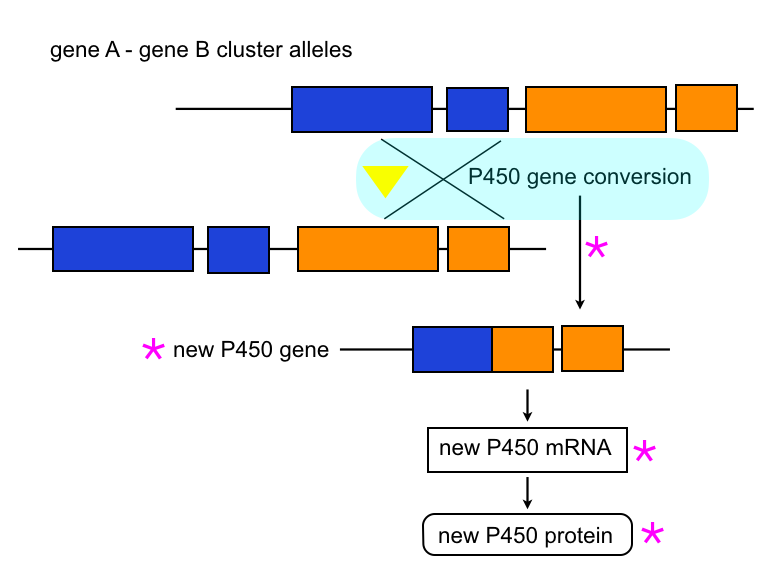
Recombination and gene conversion:
Large scale changes in P450 structure can also be obtained by gene conversion (also called non-allelic homologous recombination), where a new gene is produced, e.g. by unequal crossing-over between two alleles at a locus carrying two genes as tight P450 cluster. Examples include CYP337B3 originating from a gene conversion event between CYP337B1 and 337B2 in Helicoverpa armigera.
UP-regulation of P450 expression
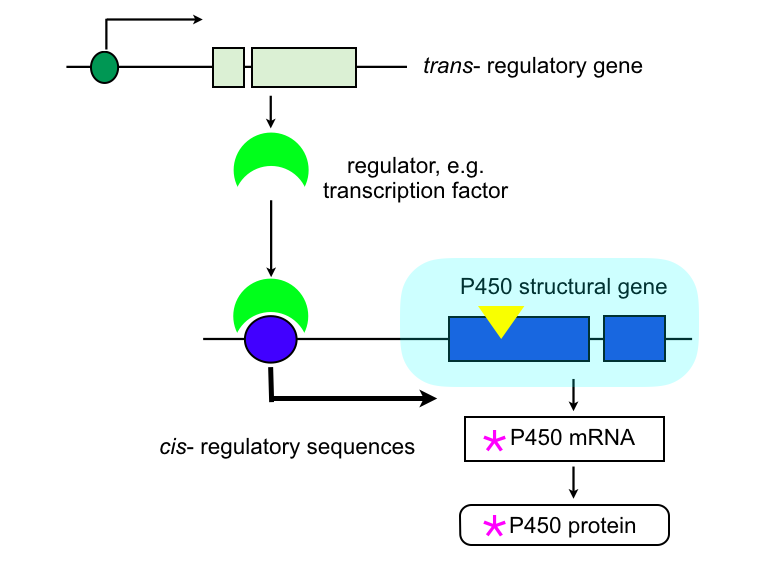
The expression of P450 genes in wild-type, susceptible populations is regulated by complex mechanisms that involve both the gene itself, and diffusible factors from elsewhere in the cell. Mutations in those mechanisms that cause constitutive UP (or DOWN) regulation of the gene can be selected, thus contributing to resistance.
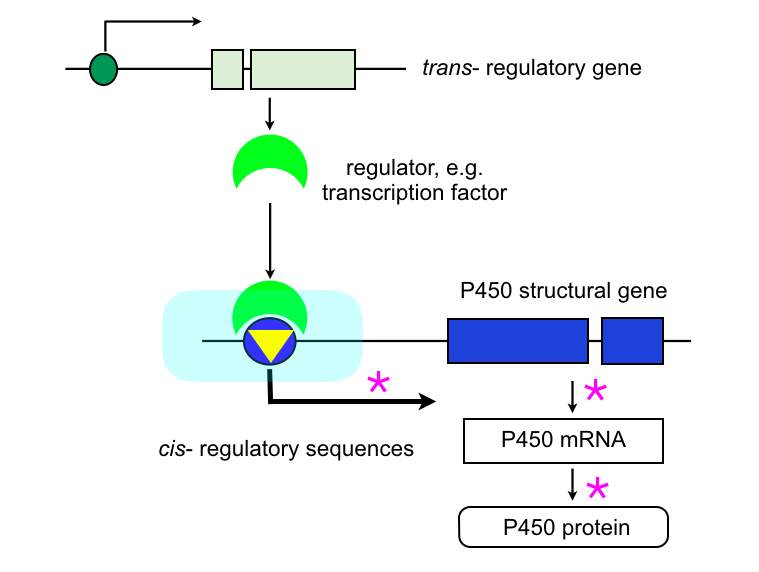
P450 overexpression in cis:
A mutation (yellow triangle) in the DNA upstream of the P450 structural gene can affect the transcriptional regulation of the gene, leading to constitutive overexpression and/or to a change in the tissue or developmental expression. This mutation can be a point mutation, indel, or insertion of a transposable element carrying regulatory sequences. The cis-mutation (Accord element insertion) in the 5‘UTR of the Cyp6g1 gene in Drosophila causing up-regulation of expression (Daborn et al., 2002) is one of the most detailed account.
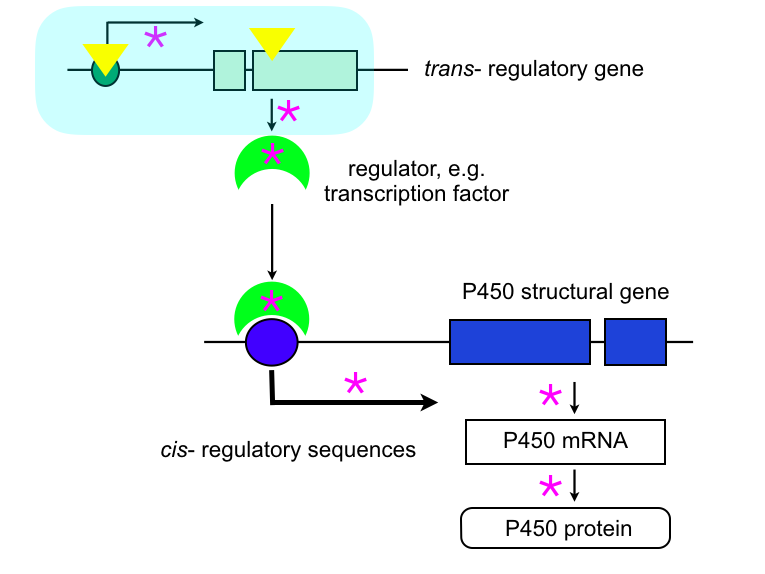
P450 overexpression in trans:
A mutation (yellow triangle) in a gene coding for a regulator of gene expression, such as a transcription factor, can affect in a cascade effect the transcriptional regulation of a P450 structural gene, leading to constitutive overexpression and/or to a change in the tissue or developmental expression. This mutation can affect the level of expression or the structure of the trans-regulator. Because the mutation is in trans, it can affect several genes, not just a single gene, and not just a P450 gene. This pleiotropic effect of a resistance mutation in trans is common. Examples include CYP6A1.
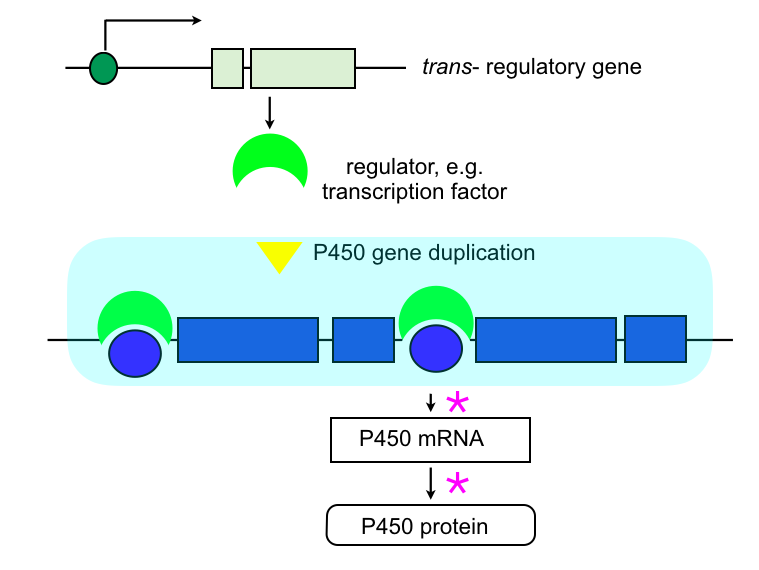
P450 duplications and amplifications:
The mutational event results in the duplication, or even amplification of one or more P450 genes, resulting in the constitutive overexpression of the gene(s). The amount of protein is not necessarily proportional to the number of copies of the duplicated gene.
DOWN-regulation of P450 expression
In the particular case of a (pro)pesticide requiring bioactivation to the active toxicant, the types of mutations described above under cis- and trans- UP-regulation can have the same (just inverse) effect. Examples include CYP4EP4 in Varroa destructor (Vlogiannitis et al., 2021). Until now, no instance of a resistance mutation causing a structural change and disruption (e.g. deletion) of a P450 gene has been observed.
Resistance case studies
A brief discussion of selected cases of P450 genes associated with insecticide resistance is provided below.